About
News and Events
Beamlines
- User Facilities
- Beamlines Directory
- BL08U1-A
- BL08U1-B
- BL09U
- BL13W1
- BL14W1
- BL14B1
- BL15U1
- BL16B1
- BL17U1
- BL01B1
- BL17B1
- BL18U1
- BL19U1
- BL19U2
Technology
- Accelerator Physics
- Accelerator Operations
- Radio Frequency
- Beam Instrumentation
- Control Systems
- Electronics & Detector
- Mechanical Engineering
- Vacuum
- Magnets
- Magnet Power Supplies
- Pulse Technique
- Cryogenics
- Front Ends
- Optics
User Information
Science and Publications
Atomic-layered Au Clusters on α-MoC as Catalysts for the Low-temperature Water-gas Shift Reaction
The water-gas shift (WGS) reaction (CO+H2O=H2+CO2) is an essential process for hydrogen generation and CO removal in various energy-related chemical operations. This equilibrium-limited reaction is favored at a low working temperature. Potential application in fuel cells also requires a WGS catalyst to be highly active, stable and energy-efficient and match the working temperature of on-site hydrogen generation and consumption units. We synthesized Au layered clusters on an α-MoC substrate to create an interfacial catalyst system for the ultra-low-temperature WGS reaction. Water was activated over α-MoC at 303 Kelvin (K), while CO adsorbed on adjacent Au sites is apt to react with surface hydroxyl groups formed from water splitting, leading to a high WGS activity at low-temperatures. Au L3 edge XAFS spectra were measured at BL14W1 beamline of Shanghai Synchrotron Radiation Facility (SSRF) and Beijing Synchrotron Radiation Facility (BSRF).
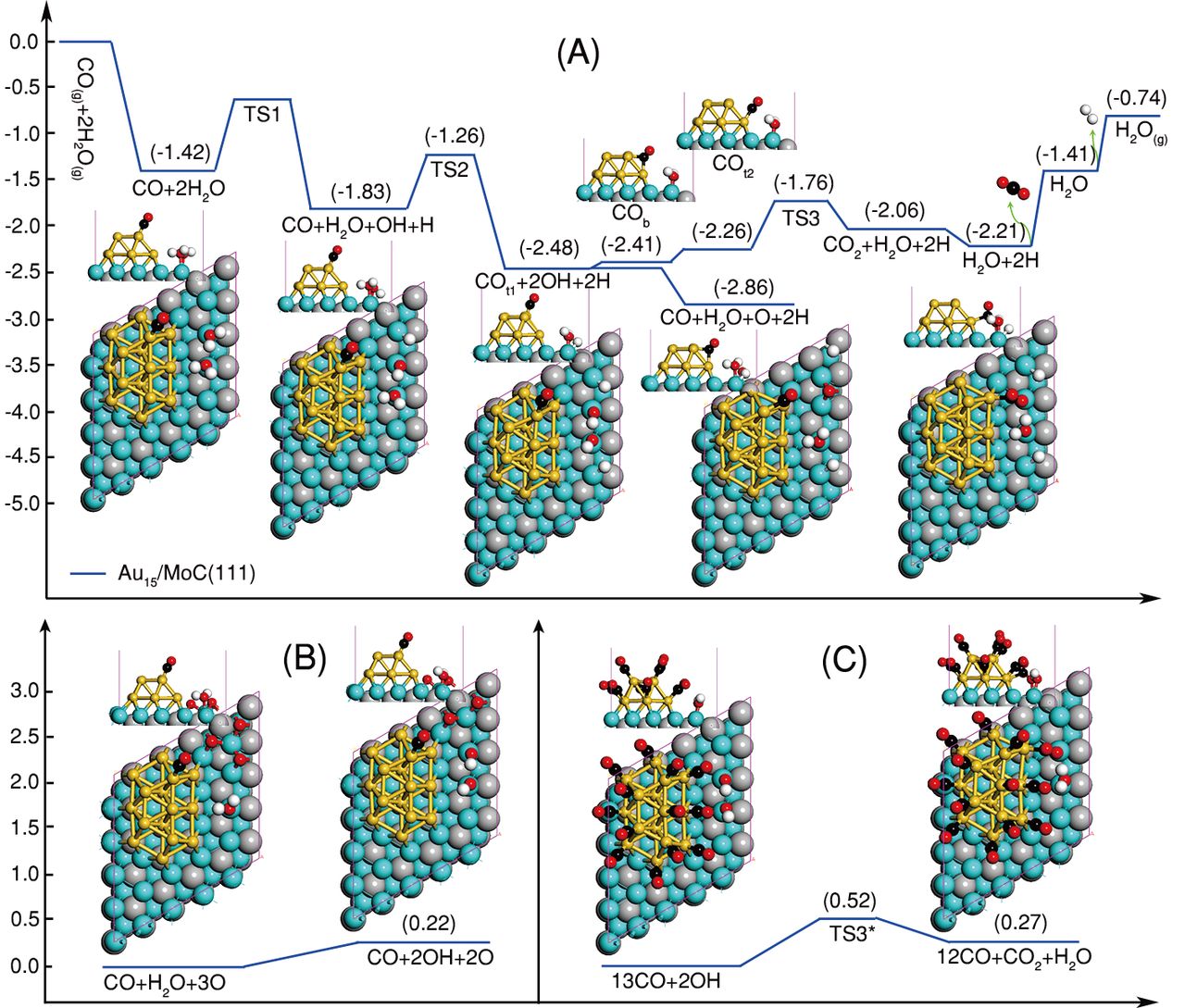
Links: DOI: 10.1126/science.aah4321


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.