About
News and Events
Beamlines
- User Facilities
- Beamlines Directory
- BL08U1-A
- BL08U1-B
- BL09U
- BL13W1
- BL14W1
- BL14B1
- BL15U1
- BL16B1
- BL17U1
- BL01B1
- BL17B1
- BL18U1
- BL19U1
- BL19U2
Technology
- Accelerator Physics
- Accelerator Operations
- Radio Frequency
- Beam Instrumentation
- Control Systems
- Electronics & Detector
- Mechanical Engineering
- Vacuum
- Magnets
- Magnet Power Supplies
- Pulse Technique
- Cryogenics
- Front Ends
- Optics
User Information
Science and Publications
Structural Determination of Catalytically Active Subnanometer Iron Oxide Clusters
26-04-2016
Supported subnanometer clusters exhibit superiority in catalytic performance compared to common nanoparticles, due to their higher fraction of exposed surfaces and larger number of active species at the metal–support interface, responding to the size effect and the support effect in heterogeneous catalysis. Here, we report the synthesis of subnanometer iron oxide clusters anchored to the surfaces of two types of ceria nanoshapes (nanorods and nanopolyhedra), as well as the structure–activity relation investigation for Fischer–Tropsch synthesis. On the basis of the comprehensive structural characterizations including aberration-corrected scanning transmission electron microscopy (STEM) and X-ray absorption fine structure (XAFS), we demonstrated that the subnanometer clusters of iron oxide are stable and catalytically active for the Fischer–Tropsch synthesis reaction. Furthermore, it is identified that finely dispersed iron oxide clusters (Fe–Ox–Fey) consisted of partially reduced Feδ+ (δ = 2.6–2.9) species in ceria nanorods are active for Fischer–Tropsch synthesis; however, another type of iron oxide cluster (Fe–Ox–Cey) composed of fully oxidized Fe3+ ions strongly interacted with the ceria nanopolyhedra support but exhibits relatively poorer activity for the reaction. These results have broad implications on the fundamental understanding of active site of supported metal catalysts at the atomic level. The ex situ X-ray absorption fine structure (XAFS) spectra of Fe K-edge (E0 = 7112 eV) for fresh and used iron−ceria samples were collected at BL14W1 beamline of Shanghai Synchrotron Radiation Facility (SSRF) or at 20-ID-B beamline of Advanced Photon Source (APS).
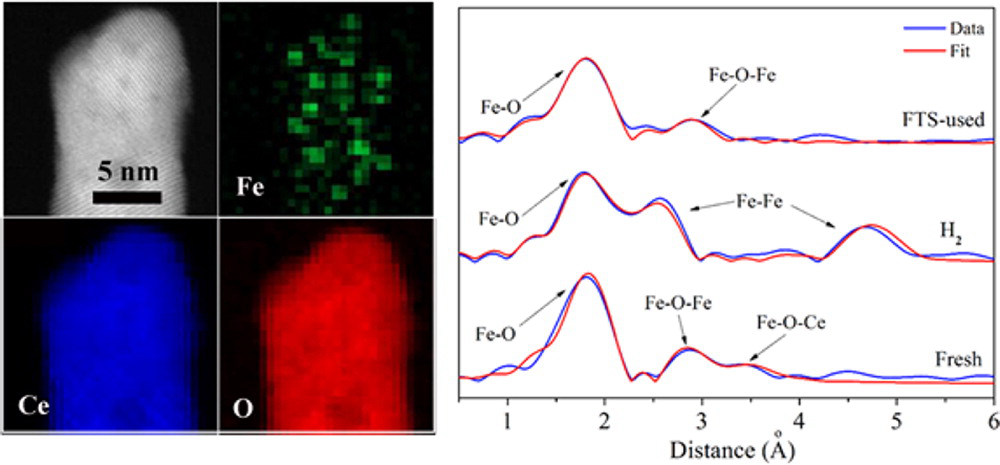
Aberration-corrected STEM-EELS results and EXAFS spectra of iron−ceria samples.


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.