About
News and Events
Beamlines
- User Facilities
- Beamlines Directory
- BL08U1-A
- BL08U1-B
- BL09U
- BL13W1
- BL14W1
- BL14B1
- BL15U1
- BL16B1
- BL17U1
- BL01B1
- BL17B1
- BL18U1
- BL19U1
- BL19U2
Technology
- Accelerator Physics
- Accelerator Operations
- Radio Frequency
- Beam Instrumentation
- Control Systems
- Electronics & Detector
- Mechanical Engineering
- Vacuum
- Magnets
- Magnet Power Supplies
- Pulse Technique
- Cryogenics
- Front Ends
- Optics
User Information
Science and Publications
Homogeneously Dispersed, Multimetal Oxygen-evolving Catalysts
08-04-2016
Earth-abundant first-row (3d) transition-metal-based catalysts have been developed for the oxygen-evolution reaction (OER); however, they operate at overpotentials significantly above thermodynamic requirements. Density functional theory suggested that non-3d high-valency metals such as tungsten can modulate 3d metal oxides, providing near-optimal adsorption energies for OER intermediates. We developed a room-temperature synthesis to produce gelled oxy-hydroxide materials with an atomically homogeneous metal distribution. These gelled FeCoW oxy-hydroxide exhibits the lowest overpotential (191 mV) reported at 10 mA per square centimeter in alkaline electrolyte. The catalyst shows no evidence of degradation following more than 500 hours of operation. X-ray absorption and computational studies reveal a synergistic interplay between W, Fe and Co in producing a favorable local coordination environment and electronic structure that enhance the energetics for OER. The in-situ Fe K-edge and Co K-edge EXAFS data collected on the BL14W1 beamline at the Shanghai Synchrotron Radiation facility (SSRF).
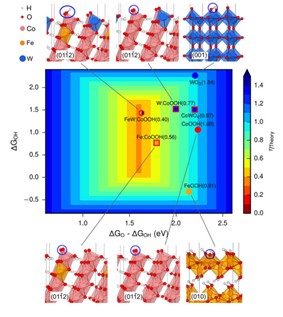
DFT+U calculated OER activities of pure and W-doped CoFe oxy-hydroxides and W oxides. The optimum is obtained for WFe-doped β-CoOOH.
Links: DOI: 10.1126/science.aaf1525


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.