About
News and Events
Beamlines
- User Facilities
- Beamlines Directory
- BL08U1-A
- BL08U1-B
- BL09U
- BL13W1
- BL14W1
- BL14B1
- BL15U1
- BL16B1
- BL17U1
- BL01B1
- BL17B1
- BL18U1
- BL19U1
- BL19U2
Technology
- Accelerator Physics
- Accelerator Operations
- Radio Frequency
- Beam Instrumentation
- Control Systems
- Electronics & Detector
- Mechanical Engineering
- Vacuum
- Magnets
- Magnet Power Supplies
- Pulse Technique
- Cryogenics
- Front Ends
- Optics
User Information
Science and Publications
Home > Science and Publications > Science Highlights
FtsZ Protofilaments Use a Hinge-Opening Mechanism for Constrictive Force Generation
24-09-2013
The essential bacterial protein FtsZ is a guanosine triphosphatase that self-assembles into a structure at the division
site termed the “Z ring”. During cytokinesis, the Z ring exerts a constrictive force on the membrane by using the
chemical energy of guanosine triphosphate hydrolysis. However, the structural basis of this constriction remains
unresolved. Here, we present the crystal structure of a guanosine diphosphate–boundMycobacterium tuberculosis
FtsZ protofilament, which exhibits a curved conformational state. The structure reveals a longitudinal interface that
is important for function. The protofilament curvature highlights a hydrolysis-dependent conformational switch at the
T3 loop that leads to longitudinal bending between subunits, which could generate sufficient force to drive cytokinesis.
The research was conducted at the SSRF beamline 17U1.
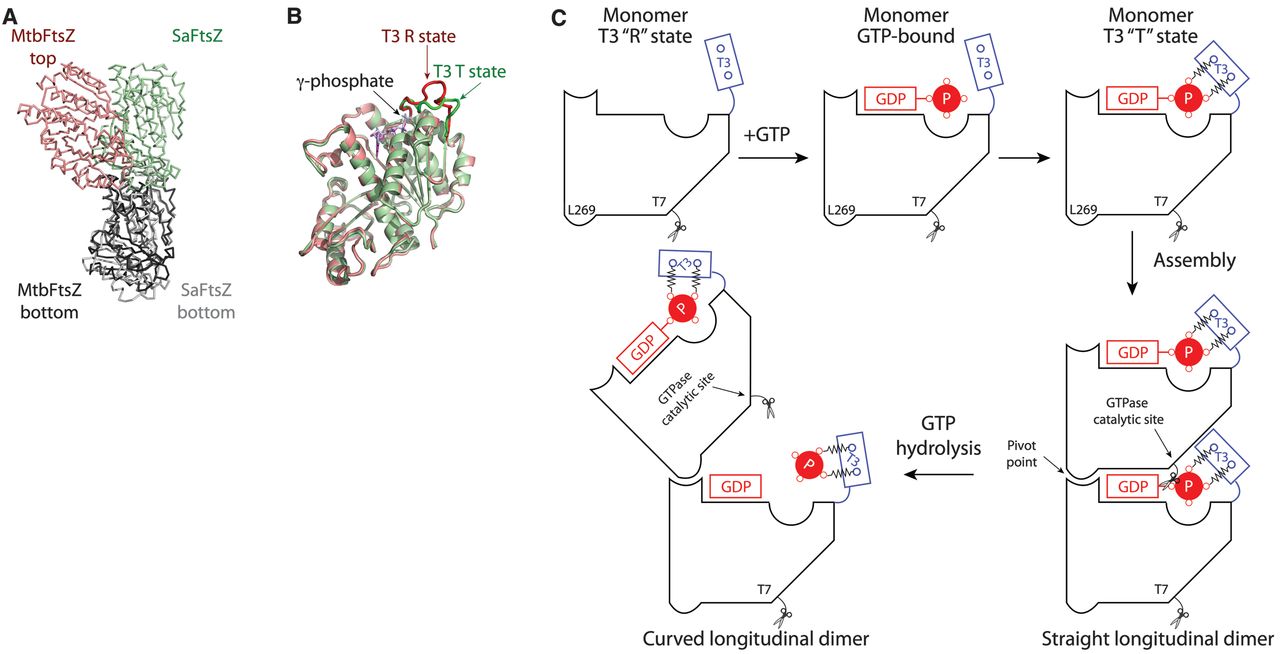
GTP hydrolysis induces a straight-to-curved conformational switch at the longitudinal interface.
相关附件


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.