About
News and Events
Beamlines
- User Facilities
- Beamlines Directory
- BL08U1-A
- BL08U1-B
- BL09U
- BL13W1
- BL14W1
- BL14B1
- BL15U1
- BL16B1
- BL17U1
- BL01B1
- BL17B1
- BL18U1
- BL19U1
- BL19U2
Technology
- Accelerator Physics
- Accelerator Operations
- Radio Frequency
- Beam Instrumentation
- Control Systems
- Electronics & Detector
- Mechanical Engineering
- Vacuum
- Magnets
- Magnet Power Supplies
- Pulse Technique
- Cryogenics
- Front Ends
- Optics
User Information
Science and Publications
Crystal Structure of a Bacterial Homologue of Glucose Transporters GLUT1–4
| 26-10-2012 |
Glucose transporters are essential for metabolism of glucose in cells of diverse organisms from microbes to humans,
exemplified by the disease-related human proteins GLUT1, 2, 3 and 4. Despite rigorous efforts, the structural
information for GLUT1–4 or their homologues remains largely unknown. Nieng Yan group of Tsinghua University
reported three related crystal structures of XylE, an Escherichia coli homologue of GLUT1–4, in complex with
D-xylose, D-glucose and 6-bromo-6-deoxy-D-glucose,the latter two were determined using MX Beamline at
Shanghai Synchrotron Radiation Facility. The structure consists of a typical major facilitator super family fold of
12transmembrane segments and a unique intracellular four-helix domain. XylE was captured in an outward-facing,
partly occluded conformation. Most of the important amino acids responsible for recognition f D-xylose or
D-glucose are invariantin GLUT1–4, suggesting functional and mechanistic conservations. Structure-based
modelling of GLUT1–4 allows mapping and interpretation of disease-related mutations.
The structural and biochemical information reported here constitutes an important framework for mechanistic
understanding of glucose transporters and sugar porters in general.
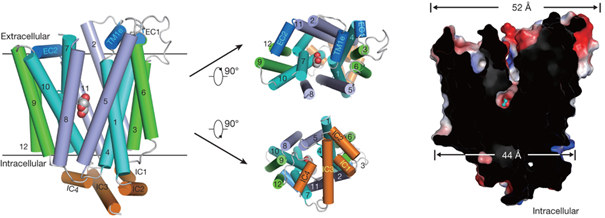
The structure of XylE
Links:http://www.nature.com/nature/journal/v490/n7420/full/nature11524.html


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.