Structure and Mechanism of a Gutamate–GABA Antiporter
11-03-2012
Enterohemmorhagic E. coli, which cause 2011 European hemorrhagic colitis outbreak, require elaborate acidresistance systems (ARs) to survive the extremely acidic environment such as the stomach (pH<2). AR2 expels intracellular protons through the decarboxylation of L-glutamate (Glu) in the cytoplasm and exchange of the reaction product c-aminobutyric acid (GABA) with extracellular Glu. The latter process is mediated by the Glu–GABA antiporter GadC. Using X-ray diffraction data from macromolecular crystallography beamline (BL17U1) of SSRF, Shi Yigong group (Tsinghua University) determined the native crystal structure of E. coli GadC under basic conditions. GadC comprises 12 transmembrane segments (TMs) and exists in a closed state, with its carboxy-terminal domain serving as a plug to block an otherwise inward-open conformation. Structural and biochemical analyses reveal the essential transport residues, identify the transport path and suggest a conserved transport mechanism involving the rigid-body rotation of a helical bundle for GadC and other amino acid antiporters.
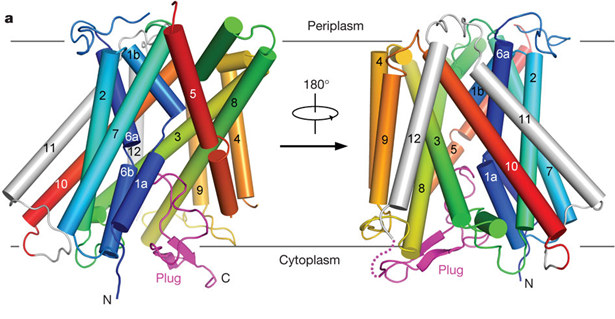
Overall structure of the WT full-length GadC. TM1–TM10 are rainbow-coloured, with TM1 in blue and TM10 in red. TM11 and TM12 are shown in grey. The C-terminal fragment (C-plug) is coloured magenta.
Links: http://www.nature.com/nature/journal/vaop/ncurrent/full/nature10917.html


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.