Structural Basis of Ultraviolet-B Perception by UVR8
29-02-2012
The Arabidopsis thaliana protein UVR8 is a photoreceptor for ultraviolet-B. Upon ultraviolet-B irradiation, UVR8 undergoes an immediate switch from homodimer to monomer, which triggers a signalling pathway for ultraviolet protection. Using X-ray diffraction data from macromolecular crystallography beamline (BL17U1) of SSRF, Shi Yigong group (Tsinghua University) determined two crystal structures of mutated UVR8 revealing a symmetric homodimer of seven-bladed β-propeller that is devoid of any external cofactor as the chromophore. Arginine residues that stabilize the homodimeric interface, principally Arg 286 and Arg 338, make elaborate intramolecular cation–π interactions with surrounding tryptophan amino acids. Two of these tryptophans, Trp 285 and Trp 233, collectively serve as the ultraviolet-B chromophore. Ultraviolet-B radiation results in destabilization of the intramolecular cation–π interactions, causing disruption of the critical intermolecular hydrogen bonds mediated by Arg 286 and Arg 338 and subsequent dissociation of the UVR8 homodimer.
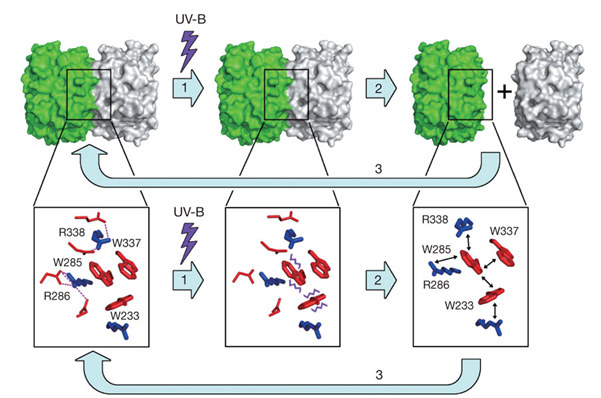
A proposed mechanism for ultraviolet-B sensing by UVR8.
Links:http://www.nature.com/nature/journal/vaop/ncurrent/full/nature10931.html


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.