About
News and Events
Beamlines
- User Facilities
- Beamlines Directory
- BL08U1-A
- BL08U1-B
- BL09U
- BL13W1
- BL14W1
- BL14B1
- BL15U1
- BL16B1
- BL17U1
- BL01B1
- BL17B1
- BL18U1
- BL19U1
- BL19U2
Technology
- Accelerator Physics
- Accelerator Operations
- Radio Frequency
- Beam Instrumentation
- Control Systems
- Electronics & Detector
- Mechanical Engineering
- Vacuum
- Magnets
- Magnet Power Supplies
- Pulse Technique
- Cryogenics
- Front Ends
- Optics
User Information
Science and Publications
SSRF User Service Brief of June
Shanghai Synchrotron Radiation Facility (SSRF) opened 16 days and provided more than 5400 hours beamtime for users in June, 2020. 235 research groups carried out 271 approved user proposals at SSRF. Users came from 102 organizations (including 59 universities, 32 research institutes, 3 hospitals, and 8 companies) and more than 600 individual users carried out experiments here.
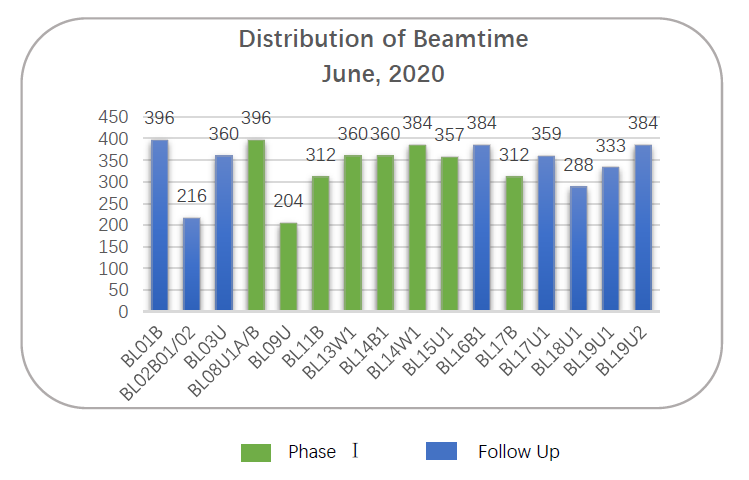
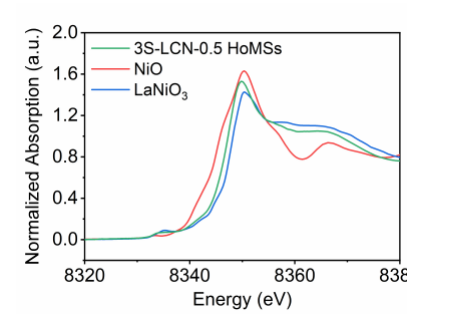
BL11B (Hard X-Ray Spectroscopy Beamline) has entered trial operation stage. So far, 13 proposal applications have been approved. In June, SSRF provided 312 hours beamtime for 7 research groups that came from 6 organizations, accounting for 5.77% of the total beamtime of June. The research team led by Dan Wang from Institute of Process Engineering, CAS, cooperating with BL11B staff, obtained a rational bottom-up design to enhance the catalytic performance through BL11B. On June 24, 2020, the team published an article titled “Dual‐Defects Adjusted Crystal Field Splitting of LaCo1‐xNixO3‐δ Hollow Multishelled Structures for Efficient Oxygen Evolution” on Angewandte Chemie International Edition. The Abstract:
“To boost the performance for various applications, a rational bottom‐up design on materials is necessary. The defect engineering on nanoparticle at the atomic level can efficiently tune the electronic behavior, which offers great opportunities in enhancing the catalytic performance. In this paper, we optimized the surface oxygen vacancy concentration and created the lattice distortion in perovskite oxide through gradient replacement of the B site with valence alternated element. The dual defects make the electron spin state transit from low spin state to high spin state, thus decreasing the charge transport resistance. Furthermore, assembly the modified nanoparticle subunits into the micro‐sized hollow multishelled structures can provide porous shells, abundant interior space and effective contact, which enables an enhanced mass transfer and a shorter charge transport path. As a result, the systemic design in the electronic and nano‐micro structures for catalyst has brought an excellent oxygen evolution performance.”
Related link: https://doi.org/10.1002/anie.202007077
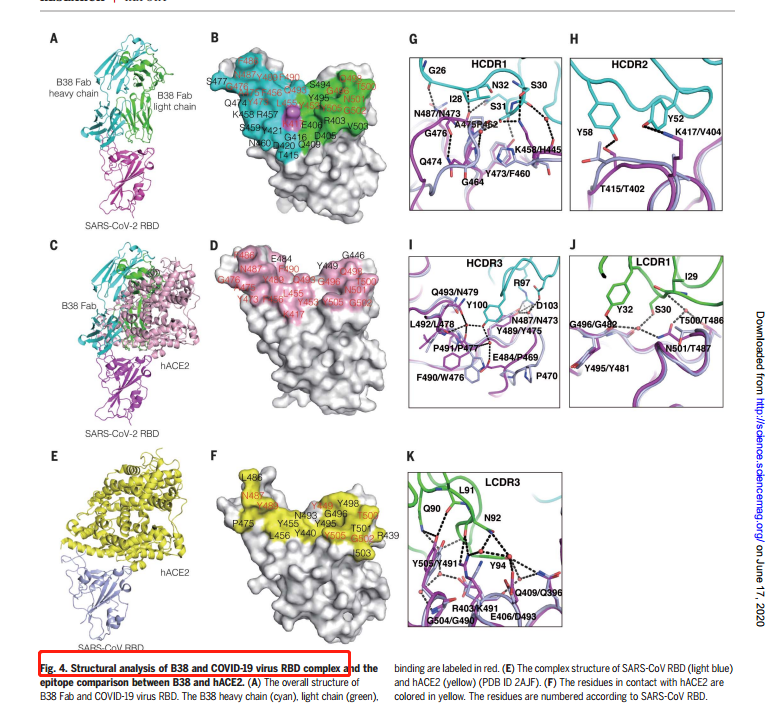
In addition, on June 12, 2020, the research team led by Fu Gao at Institute of Microbiology, CAS, published an article titled with “A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2” on Nature, indicating the blocking effect and neutralizing abilities of RBD-B38. The data were collected at BL17U1. The Abstract:
“Neutralizing antibodies could potentially be used as antivirals against the coronavirus disease 2019(COVID-19) pandemic. Here, we report isolation of four human-origin monoclonal antibodies from aconvalescent patient, all of which display neutralization abilities. The antibodies B38 and H4 block binding between the spike glycoprotein receptor binding domain (RBD) of the virus and the cellular receptor angiotensin-converting enzyme 2 (ACE2). A competition assay indicated different epitopes on the RBD for these two antibodies, making them a potentially promising virus-targeting monoclonal antibody pair for avoiding immune escape in future clinical applications. Moreover, a therapeutic study in a mouse model validated that these antibodies can reduce virus titers in infected lungs. The RBD-B38 complex structure revealed that most residues on the epitope overlap with the RBD-ACE2 binding interface, explaining the blocking effect and neutralizing capacity. Our results highlight the promise of antibody-based therapeutics and provide a structural basis for rational vaccine design.”
Related link: http://science.sciencemag.org/content/368/6496/1274


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.