About
News and Events
Beamlines
- User Facilities
- Beamlines Directory
- BL08U1-A
- BL08U1-B
- BL09U
- BL13W1
- BL14W1
- BL14B1
- BL15U1
- BL16B1
- BL17U1
- BL01B1
- BL17B1
- BL18U1
- BL19U1
- BL19U2
Technology
- Accelerator Physics
- Accelerator Operations
- Radio Frequency
- Beam Instrumentation
- Control Systems
- Electronics & Detector
- Mechanical Engineering
- Vacuum
- Magnets
- Magnet Power Supplies
- Pulse Technique
- Cryogenics
- Front Ends
- Optics
User Information
Science and Publications
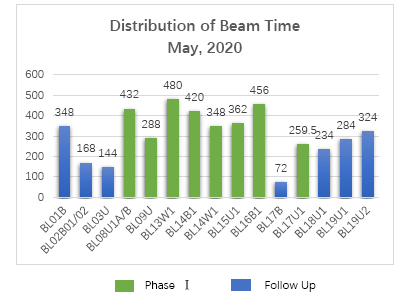
SSRF User Service Brief of May
Shanghai Synchrotron Radiation Facility (SSRF) opened 19 days and provided more than 4600 hours beamtime for users in May, 2020. 190 research groups carried out 222 approved user proposals at SSRF. Users came from 96 organizations (including 48 universities, 30 research institutes, 6 hospitals, and 12 companies) and more than 460 individual users carried out experiments here.
Since the outbreak of COVID-19, SSRF has continuously assisted several research teams carrying out studies related to COVID-19. The main achievements are as follows.
1. The research team led by Xinquan Wang and Linqi Zhang at Tsinghua University obtained the crystal structure of antibody-RBD complex through SSRF BL17U1. On May 26, 2020, the team published an article titled with “Human neutralizing antibodies elicited by SARS-CoV-2 infection” on Natural. The Abstract:
“The emerging coronavirus SARS-CoV-2 pandemic presents a global health emergency in urgent need of interventions1–3. SARS-CoV-2 entry into the target cells depends on binding between the receptor-binding domain (RBD) of the viral Spike protein and the ACE2 cell receptor2,4–6. Here, we report the isolation and characterization of 206 RBD-specific monoclonal antibodies derived from single B cells of eight SARS-CoV-2 infected individuals. We identified antibodies with potent anti-SARS-CoV-2 neutralization activity that correlates with their competitive capacity with ACE2 for RBD binding. Surprisingly, neither the anti-SARS-CoV-2 antibodies nor the infected plasma cross-reacted with SARS-CoV or MERS-CoV RBDs, although substantial plasma cross-reactivity to their trimeric Spike proteins was found. Crystal structure analysis of RBD-bound antibody revealed steric hindrance that inhibits viral engagement with ACE2 and thereby blocks viral entry. These findings suggest that anti-RBD antibodies are viral species-specific inhibitors. The antibodies identified here may be candidates for the development of SARS-CoV-2 clinical interventions.”
Related link: https://www.nature.com/articles/s41586-020-2380-z
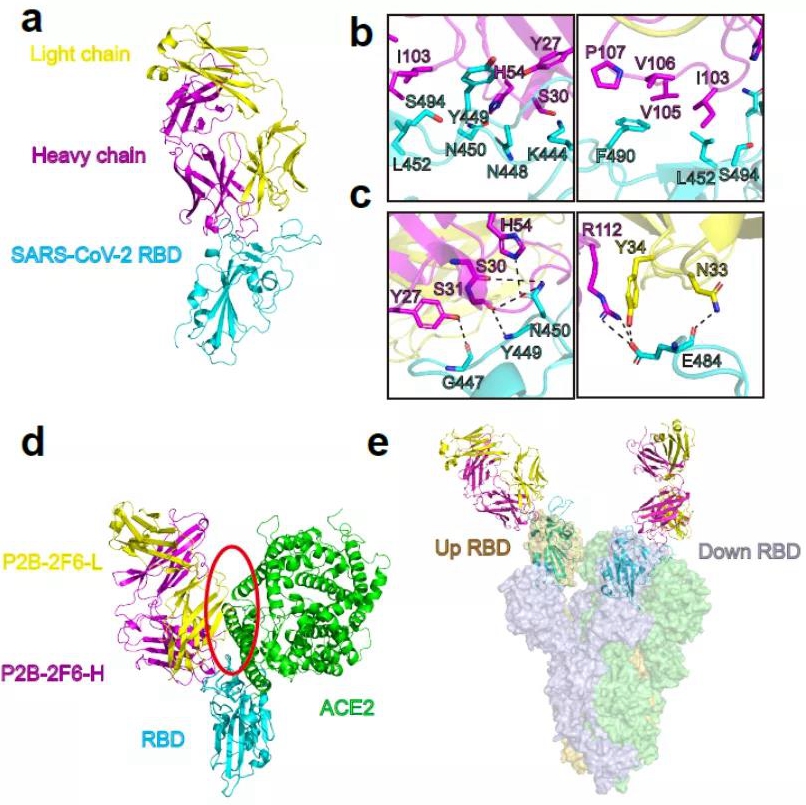
Crystal Structure of P2B-2F6 in complex with SARS-CoV-2RBD
2. The research team led by Jinghua Yan at CAS Key Laboratory of Microbial Physiological and Metabolic Engineering, Institute of Microbiology, CAS, collected high resolution crystal structure of antibody SARS-Cov-2 S protein complex through SSRF BL17U1. On May 26, the team published an article titled with “A human neutralizing antibody targets the receptor binding site of SARS-CoV-2” on Natural. The Abstract:
“An outbreak of the coronavirus disease 2019 (COVID-19)1–3, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)4 spread globally. Countermeasures are needed to treat and prevent further dissemination of the virus. In this study, we report the isolation of 2 specific human monoclonal antibodies (MAbs) from a convalescent COVID-19 patient. CA1 and CB6 demonstrated potent SARS-CoV-2-specific neutralization activity in vitro against SARS-CoV-2. In addition, CB6 inhibited SARS-CoV-2 infection in rhesus monkeys at both prophylactic and treatment settings. Further structural studies revealed that CB6 recognizes an epitope that overlaps with angiotensin converting enzyme 2 (ACE2)-binding sites in SARS-CoV-2 receptor binding domain (RBD), thereby interfering with the virus/receptor interactions by both steric hindrance and direct interface-residue competition. Our results suggest CB6 deserves further clinical translation.”
Related link: https://doi.org/10.1038/s41586-020-2381-y

The crystal structure of CB6 and SARS-CoV-2-RBD complex and the competitive binding of CB6 and hACE2 with SARS-CoV-2-RBD


 Copyright©2006.12 Shanghai Advanced Research Institute.
Copyright©2006.12 Shanghai Advanced Research Institute.