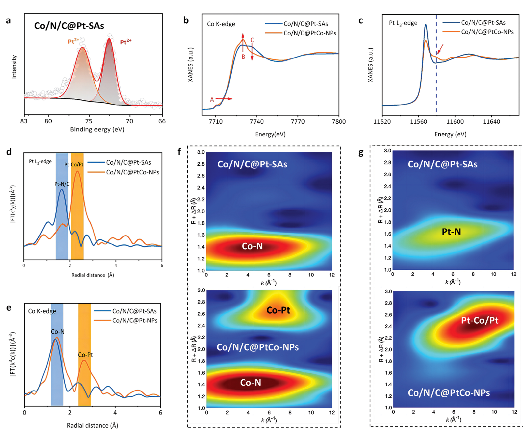
Researchers employed the BL17B synchrotron-based XAFS technique to observe the transfer of cobalt (Co) electrons to platinum (Pt) atoms in Co/N/C@PtCo-NPs, confirming the existence of Co-Pt bonds. This identification establishes the CoPt nanoparticle as a site for enhancing oxygen reduction reaction (ORR). Their findings provide crucial evidence for understanding the structure-function relationship and catalytic mechanism of composite low Pt-group-metal / emerging PGM-free catalyst in ORR.
Decoupling the Synergy Between PGM and PGM-Free Moieties toward Oxygen Reduction Reaction
Scientific Achievement
The research team from Guangzhou University, led by Ye Siyu and Du Lei, in collaboration with Wang Liguang's team from Zhejiang University, synthesized two model catalysts: atomically dispersed Co/N/C supporting Pt single atoms (Co/N/C@Pt-SAs) and PtCo nanoparticles (Co/N/C@PtCo-NPs). Interestingly, the Co/N/C@PtCo-NPs catalyst presents higher ORR mass activity and stability prior to Co/N/C@Pt-SAs. The researchers, through a combination of physical characterization and theoretical calculations, unveiled the positive impact of dual “built-in electric field” and "pinning effects" in the composite catalysts on the ORR process. Their findings were published in Small in March 2024 under the title "Decoupling the Synergy Between PGM and PGM-Free Moieties toward Oxygen Reduction Reaction.
Significance and Impact
The investigation of the synergistic effect in LP/PF catalysts, exemplified by the Co/N/C@PtCo-NPs catalyst, offers deeper insights into the understanding of emerging LP/PF catalysts and is providing guides for the development of oxygen reduction electrocatalysts.
Research background
Cathodic oxygen reduction reaction (ORR) is fundamental for electrochemical energy conversion and storage applications such as metal-air batteries and fuel cells. Recently, coupling the conventional low Pt-group-metal (low-PGM, LP) and emerging PGM-free (PF) moiety to form a composite LP/PF catalyst is proposed to be an advanced strategy to improve the intrinsic activity and stability of oxygen reduction reaction (ORR) catalysts. However, the specific synergy between LP and PF moieties has not been well elucidated, particularly for bimetallic PtM NPs on M/N/C.
Main research contents
To obtain insights into the underlying reasons for the activity and stability enhancement of LP/PF catalysts, researchers synthesized two model catalysts using the mature Co/N/C as the substrate to load Pt single atoms (Co/N/C@Pt-SAs) and PtCo nanoparticles (Co/N/C@PtCo-NPs), respectively. Interestingly, the Co/N/C@PtCo-NPs has better intrinsic ORR activity and stability than Co/N/C@Pt-SAs.
In order to figure out the mechanism of performance improvement, researchers have analyzed their electronic structures though physical characterization and theoretical calculation, such as XAFS, XPS, UPS, XAFS experiments and DFT calculation. The specific synergy in Co/N/C@PtCo-NPs is due to the dual “built-in electric field”: one with a direction from Pt to neighboring Co atoms in PtCo NPs, another from Pt to CoNx moieties, leading to a localized asymmetric electron cloud, well-regulated ad-sorption/desorption of key oxygen-containing species and thus the enhanced intrinsic ORR activity. Besides, the presence of Co can reduce the overall oxidation state of PtCo NPs and en-hance the interaction between PtCo NPs and Co/N/C, thus stabilizing PtCo NPs, i.e., the “pinning effect”. These results pro-vide more insights into the understanding of emerging LP/PF catalysts.
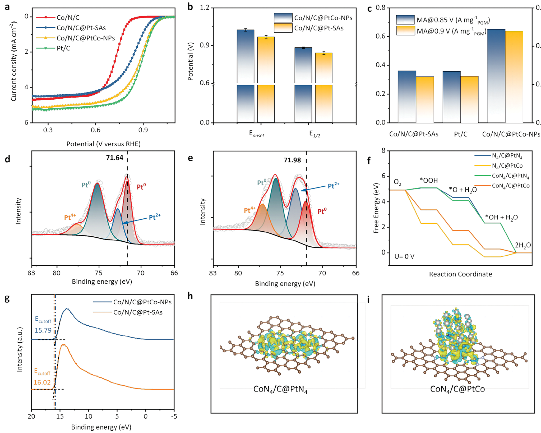
Fig. 1. ORR activity and theoretical analysis
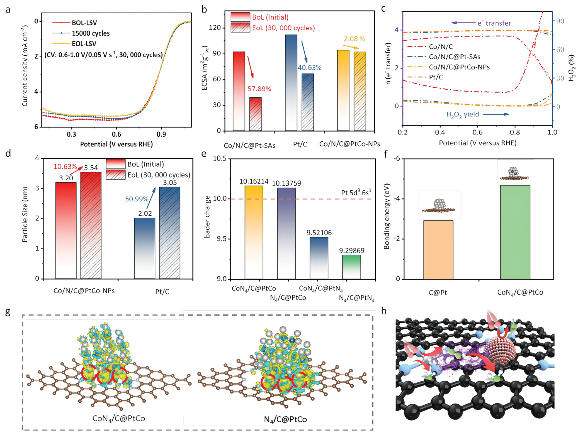
Fig. 2. Stability tests and the strong interfacial interactions.
附件下载:
