Title: Peng X, Yang L, Liu Z, Lou S, Mei S, Li M, Chen Z, Zhang H. Structural basis for recognition of antihistamine drug by human histamine receptor. Nat Commun. 2022 Oct 15;13(1):6105. doi: 10.1038/s41467-022-33880-y. PMID: 36243875; PMCID: PMC9569329.
By Zhang H etc, School of Pharmacy, Zhejiang University
The histamine receptors belong to the G protein-coupled receptor (GPCR) superfamily, and play important roles in the regulation of histamine and other neurotransmitters in the central nervous system, as potential targets for the treatment of neurologic and psychiatric disorders. Here we report the crystal structure of human histamine receptor H3R bound to an antagonist PF-03654746 at 2.6 Å resolution. Combined with the computational and functional assays, our structure reveals binding modes of the antagonist and allosteric cholesterol. Molecular dynamic simulations and molecular docking of different antihistamines further elucidate the conserved ligand-binding modes. These findings are therefore expected to facilitate the structure-based design of novel antihistamines.
Publications
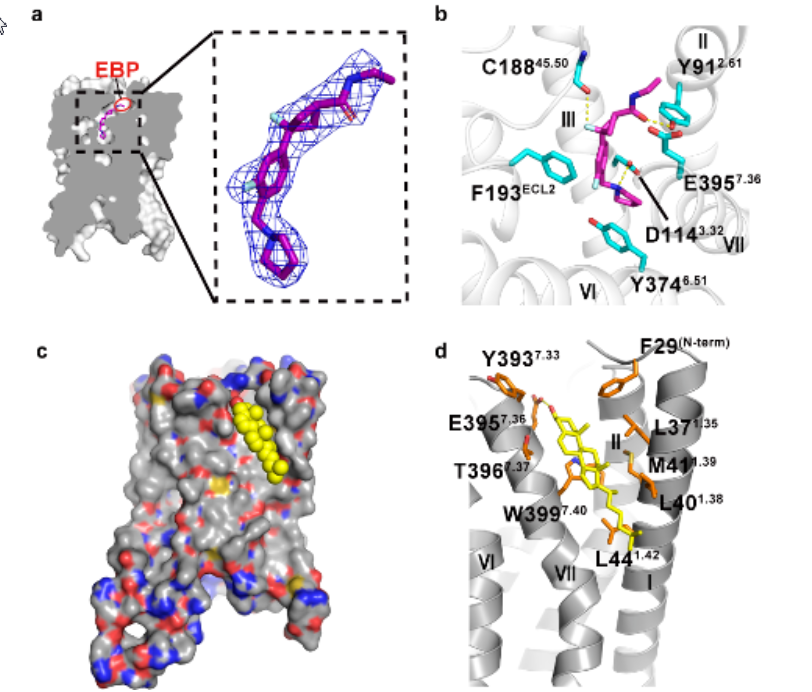
The molecular mechanism of PF-03654746 and chelestrol interacting with H3R
附件下载:
